- English
- 中文


Professionalism and high requirements, our company intent to create the perfect product to satisfy customers demands.


We consistently use raw materials from universally known manufacturers which have passed inspection


our company has continuously introduced automated equipment and high quality machines
Our senior management personnel have engaged in design and production of First Aid and Health Care products for the past 14 years. Thus they have an abundance of experience. In product manufacturing, we pay close attention to the practicality of each product to meet our customer requirements at the largest extent.


It was formally established in March 2010. At the beginning of its establishment, GFA obtained the first-class medical device production registration certificate and the second-class medical device business registration certificate.
Since GFA was founded, we continuously carry out standardization work, and have established and passed quality systems such as ISO9001, ISO13485, CMDR, MDD93/42/EEC, QSR 820 and 21CFR210/211.
In September 2013 and December 2016, our company passed the device inspections of the FDA, and passed the drug inspections of the FDA in November 2014, December 2016 and April 2019.

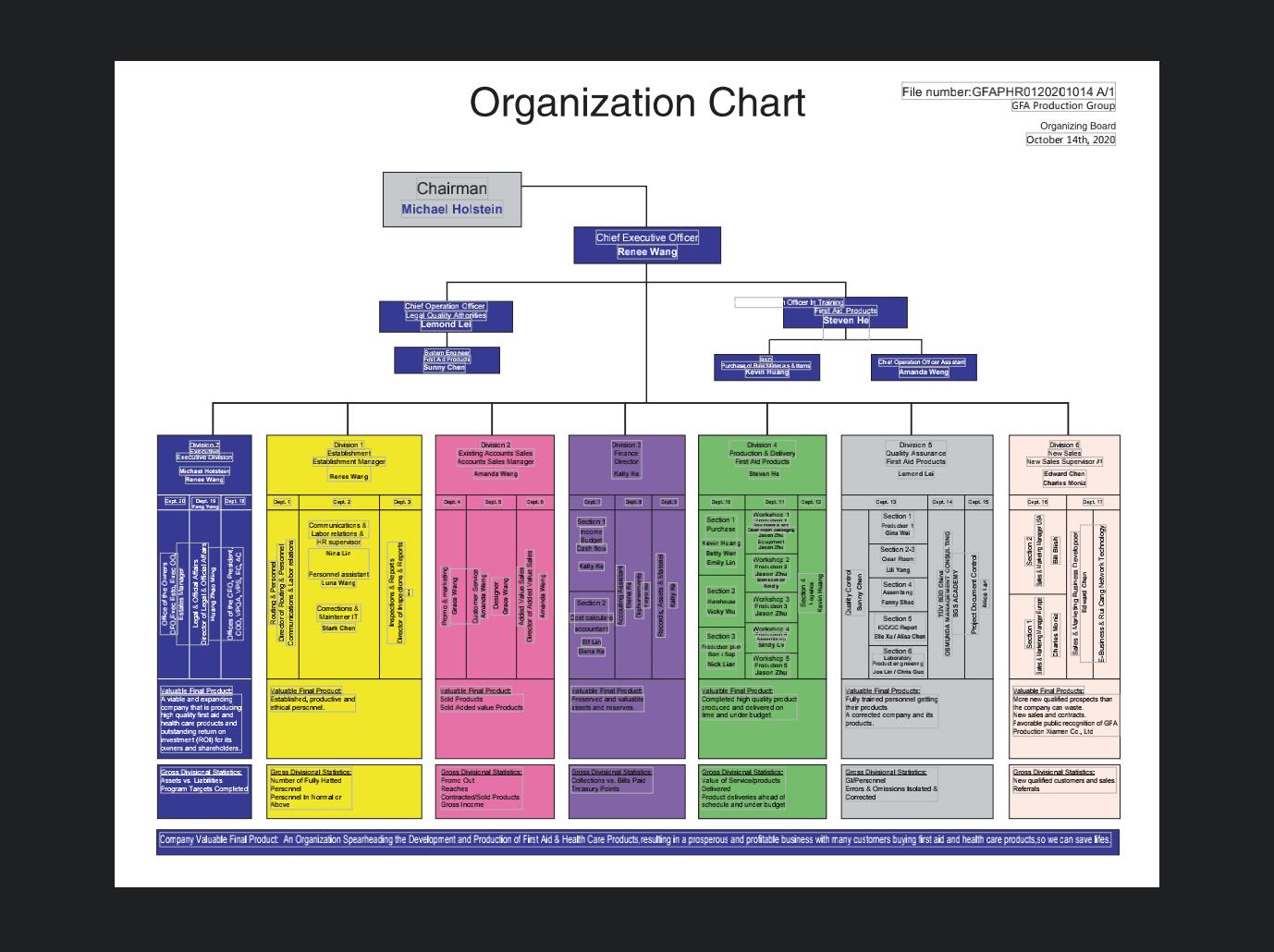
Running and coordinate the company as well as formulate development strategies, integrating all available resources to ensure operates smoothly and effectively as an entirety.


In charge of Quality Assurance and Legal Affair Apartment, coordinating a quality control team and taking care of product inspection, aiming at making sure products safe and reliable.


Taking responsibility for directing, conduct regular training, development programs.Customized for customers Practical, innovative and tailored first aid training.